| http://1048believe.com/wp-admin/js/specific-heat-capacity-formula-physics-i0.jpg |
Measuring Energy Changes
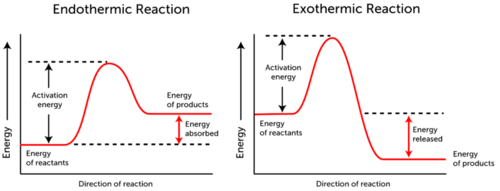 |
| https://dr282zn36sxxg.cloudfront.net/datastreams/f-d%3Adf0a2687d885c997ec852a60b09181c51b0a234ada913 6e0288d4e8c%2BIMAGE_THUMB_POSTCARD%2BIMAGE_THUMB_POSTCARD.1 |
| http://www.sartep.com/chem/images/calorieconvert.gif |
Types of Energy
- Kinetic Energy- energy of motion
- Radiant Energy- energy from the sun or solar energy
- Thermal Energy- energy associated with the random motion of atoms and molecules
- Chemical Energy- energy stored within the structural units of chemical substances
- Potential Energy- energy stored or energy of position
No comments:
Post a Comment