Today we learned the difference between acids and bases; everything from the physical characteristics to their chemical makeup. We also learned the difference between Arrhenius and Bronsted-Lowery bases and acids. Below is a quick overview of what we learned:
Physical Properties
- Acids- taste sour, feel sticky, show red or pink on litmus paper
- Bases- taste bitter, feel slippery, show blue on litmus paper
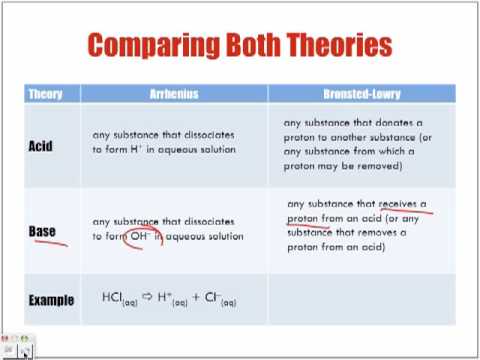
Arrhenius Acids and Bases
 |
| http://img.youtube.com/vi/ph5lqm5fi8s/0.jpg |
- Acids- produce hydrogen ions in solution (H+) [HCL--> H+ + Cl-]
- Bases- produce hydroxide ions in solution (OH-) [NaOH--> Na+ + OH-]
- Amphitheater substance- can be an acid or a base [water]
Bronsted-Lowery Acids and Bases
- Acids- donate a proton (H+)
- Bases- accept a proton (H+)
- Conjugate acid- substance formed when a proton is added to a base
- Conjugate base- remaining substance when a proton is lost from an acid
******* Acids produce conjugate bases, Bases produce conjugate acids********
Acid Strength
- List of Strong Acids
- Perchloric acid (HClO4)
- Chloric acid (HClO3)
- Hydrochloric acid (HCl)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
- Nitric acid (HNO3)
- Sulfuric acid (H2SO4)
If the oxygen outnumbers the hydrogen by two or more, it is considered a strong acid.
For more information or practice use the links below:
Detailed Explanation with Examples
Video explanation
Shorter video
Quiz Yourself