Physical Properties
- Acids- taste sour, feel sticky, show red or pink on litmus paper
- Bases- taste bitter, feel slippery, show blue on litmus paper
 |
| http://img.youtube.com/vi/ph5lqm5fi8s/0.jpg |
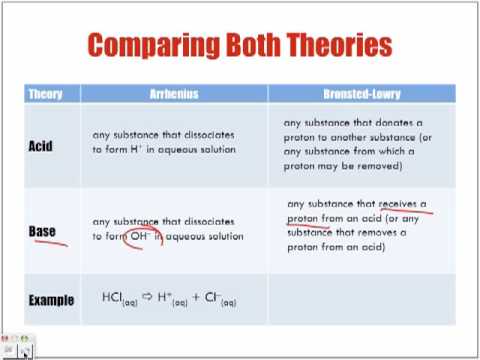
- Acids- produce hydrogen ions in solution (H+) [HCL--> H+ + Cl-]
- Bases- produce hydroxide ions in solution (OH-) [NaOH--> Na+ + OH-]
- Amphitheater substance- can be an acid or a base [water]
- Acids- donate a proton (H+)
- Bases- accept a proton (H+)
- Conjugate acid- substance formed when a proton is added to a base
- Conjugate base- remaining substance when a proton is lost from an acid
Acid Strength
- List of Strong Acids
- Perchloric acid (HClO4)
- Chloric acid (HClO3)
- Hydrochloric acid (HCl)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
- Nitric acid (HNO3)
- Sulfuric acid (H2SO4)
For more information or practice use the links below:
Detailed Explanation with Examples
Video explanation
Shorter video
Quiz Yourself
Thank you for the summary of the lesson and the links. Some of the links really helped me on the quiz we took today!
ReplyDeleteThat is a very deep lecture, Megan. I really could use these before the tests. It may help save my grade. You really understand this lesson. If I need any help, I will ask you. I just wish I saw these before the quiz.
ReplyDeleteIf the products you look for are not in our catalog we would be pleased to offer our custom synthesis service. DAnF6Pye
ReplyDelete