- Places 100.mL of room temp. distilled water into a Styrofoam cup and recorded temp.
- Find the mass of the test tube
- Place metal in test tube about 1/3 of the way
- Find the mass of the test tube with metal
- Heat the metal up in a water bath to boiling. Let boil for 5 minutes then record the temperature of the water (the metal should be the same temp.)
- Pour the metal into the water with the Styrofoam cup. Place probe in cup and stir
- Record the highest temperature reached by the water
- mass of test tube- 15.9355
- mass of test tube and metal- 54.9484
- initial temperature of metal- 100.1 oC
- initial temperature of H20- 23.4 oC
- final temperature of metal and H20- 27.5 oC
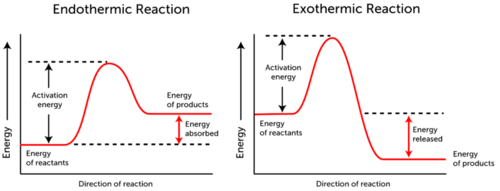
Overview or explanation of lab
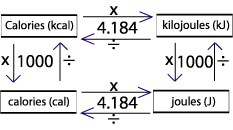
How to calculate specific heat