Physical Properties
- Acids- taste sour, feel sticky, show red or pink on litmus paper
- Bases- taste bitter, feel slippery, show blue on litmus paper
 |
| http://img.youtube.com/vi/ph5lqm5fi8s/0.jpg |
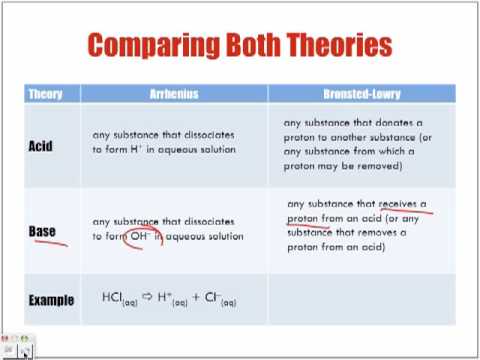
- Acids- produce hydrogen ions in solution (H+) [HCL--> H+ + Cl-]
- Bases- produce hydroxide ions in solution (OH-) [NaOH--> Na+ + OH-]
- Amphitheater substance- can be an acid or a base [water]
- Acids- donate a proton (H+)
- Bases- accept a proton (H+)
- Conjugate acid- substance formed when a proton is added to a base
- Conjugate base- remaining substance when a proton is lost from an acid
Acid Strength
- List of Strong Acids
- Perchloric acid (HClO4)
- Chloric acid (HClO3)
- Hydrochloric acid (HCl)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
- Nitric acid (HNO3)
- Sulfuric acid (H2SO4)
For more information or practice use the links below:
Detailed Explanation with Examples
Video explanation
Shorter video
Quiz Yourself



