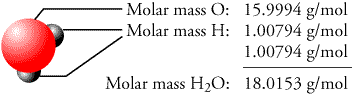 |
| http://preparatorychemistry.com/images/H2O_space_atomic_mass_CS.gif |
Step 1: Figure out what unit you are in and what you have to convert to. (ex. moles--> mass)
Step 2: See if there are any compounds in the problem. If there are, then you have to convert the mass into molar mass before you convert to moles. (Look at second set of steps for instruction on how to do this)
Step 3: Convert
Step 4: Simplify looking at sig figs
Molar Mass: (look at example if you don't understand)
1. Figure out the Chemical formula
2. Break apart each element and find out their atomic mass by looking at Periodic Table
3. Multiply the amount of atoms by the atomic mass for that specific element
4. Add up all the atoms's masses to get the molar mass
For example:
What is the molar mass of Magnesium Chloride?
1) MgCl2
3) Magnesium: (1*24.31) Chloride: (2*35.45)
4. 24.31+70.90=95.11 g/mol MgCl2
*****Hints*******
- There will always be two decimal places in Molar Mass measurements
- Sig figs still apply when simplifying
- Molar Mass just replaces atomic mass in the mole roadmap equations
OCC.edu is if you don't understand the lesson from my notes above or if you need a little more information
ths.sps.lane is a quick quiz to see how much you know
No comments:
Post a Comment